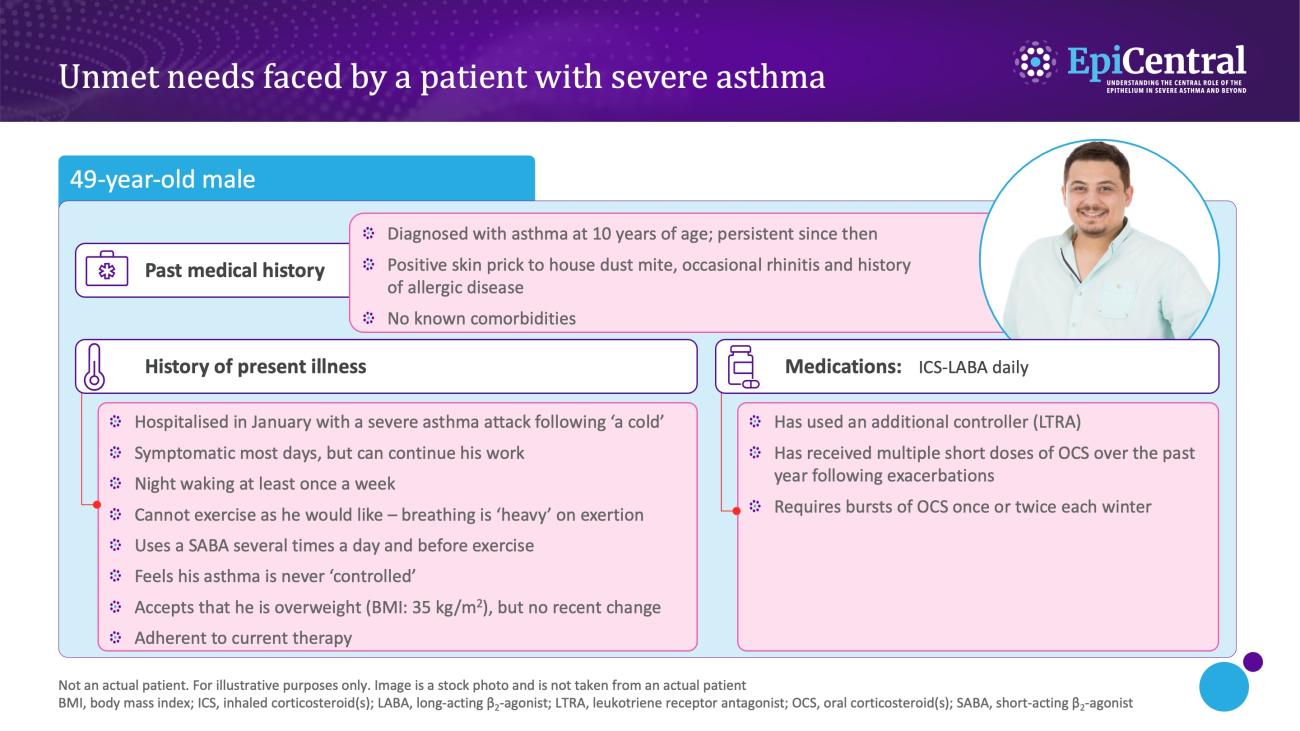
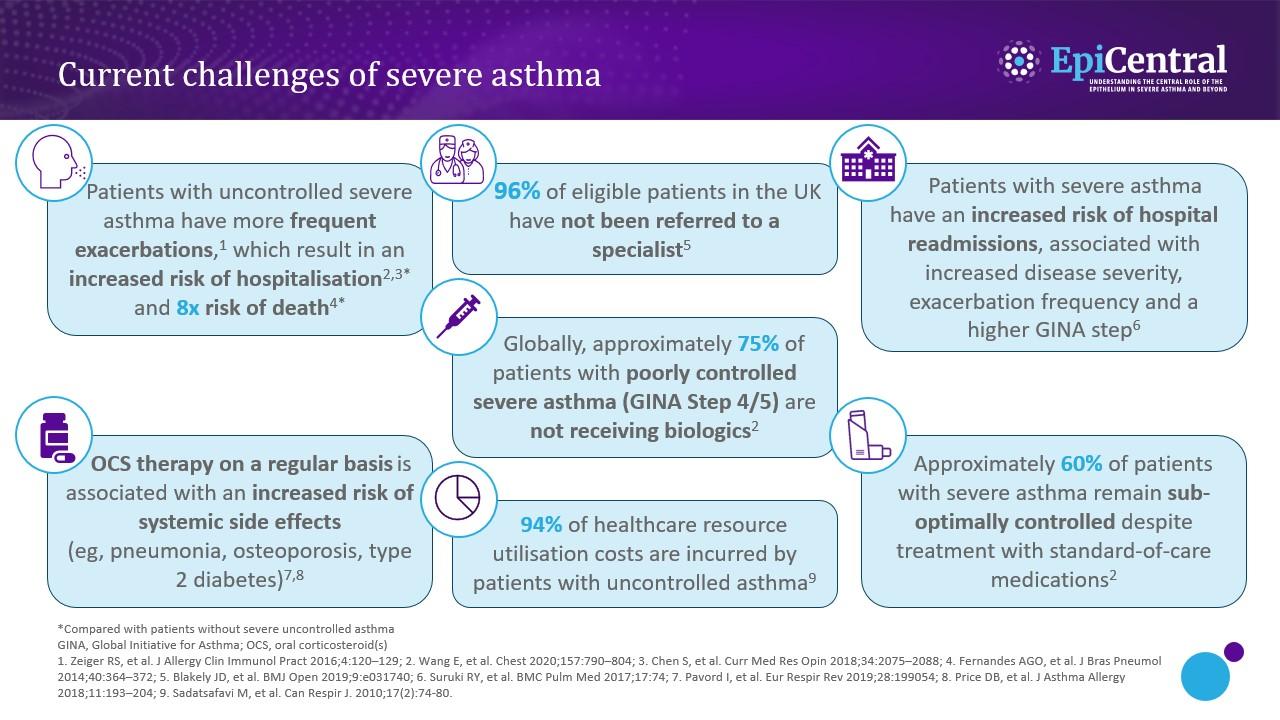
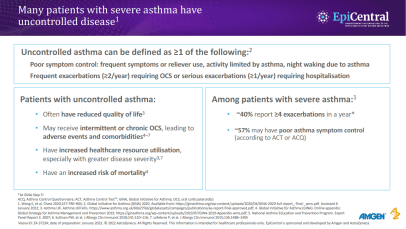
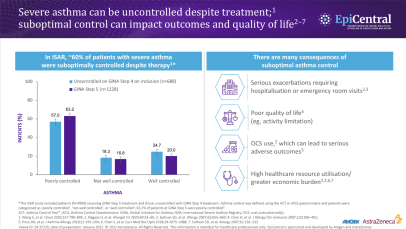
References
1. Caminati M, et al. J Asthma Allergy. 2021;14:457–466;
2. Global Asthma Network. The global asthma report 2022. Available from: http://globalasthmareport.org/resources/Global_Asthma_Report_2022.pdf (Accessed August 2024);
3. Rogliani P, et al. Pulm Ther. 2020;6:47–66;
4. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2024. Available from: https://ginasthma.org/2024-report/ (Accessed May 2024);
5. Blakely JD, et al. BMJ Open. 2019;9:e031740;
6. Price D, et al. J Asthma Allergy. 2017;10:209-223;
7. Hardtstock F, et al. J Asthma. 2023;60(7):1280-1289;
8. Castillo JR, et al. J Allergy Clin Immunol Pract. 2017;5(4):918-927;
9. Murphy KR, et al. J Allergy Clin Immunol Pract. 2020;8(7):2263-2274.e5;
10. Beuther DA, et al. J Allergy Clin Immunol Pract. 2022;10(12):3204-3212.e2;
11. Dhruve H, et al. Thorax. 2023;78:A66;
12. Charriot J, et al. Eur Respir Rev. 2016;25:77–92;
13. Zervas E, et al. ERJ Open Res. 2018;4:00125–02017;
14. Djukanovic R, et al. Eur Respir J. 2018;52:1801671;
15. Wang E, et al. Chest. 2020;157:790–804;
16. Sadatsafavi M, et al. Can Respir J. 2010;17(2):74-80;
17. Suruki RY, et al. BMC Pulm Med. 2017;17:74;
18. Mayers I, et al. Eur Respir J. 2022;60(Suppl66):2306;
19. Halner A, et al. PLoS One. 2021;16:e0254425;
20. Soong W, et al. Ann Allergy Asthma Immunol. 2020;125:S27 (Abstract P203);
21. Ambrose CS, et al. Pragmat Obs Res. 2020;11:77–90;
22. Jackson DJ, Bacharier LB. Ann Allergy Asthma Immunol. 2021;127(5):524-529;
23. Pavord ID. Curr Opin Pulm Med. 2019;25:51–58;
24. Price DB, et al. J Asthma Allergy. 2018;11:193–204;
25. Chen H, et al. J Allergy Clin Immunol. 2007;120:396–402;
26. Stubbs MA, et al. J Asthma Allergy. 2021;14:1527–1537;
27. Busse WW, Kraft M. Eur Respir Rev. 2022;31:210176;
28. Chen S, et al. Curr Med Res Opin. 2018;34:2075–2088;
29. Nunes C, et al. Asthma Res Pract. 2017;3:1;
30. Usmani OS, Levy ML. NPJ Prim Care Respir Med. 2023;33(1):24;
31. Tran TN, et al, ATS abstract 2024;
32. Chung LP, et al. Respirology. 2020;25:161–172;
33. Papapostolou G, et al. Eur Clin Respir J. 2020;8:1856024;
34. Bleecker ER, et al. Am J Respir Crit Care Med. 2020;201:276–293;
35. Canonica GW, et al. World Allergy Organ J. 2019;12:100007;
36. Sweeney J, et al. Thorax. 2016;71:339–346;
37. Scelo G, et al. Ann Allergy Asthma Immunol. 2024;132(1):42-53;
38. Skov IR, et al. Eur Respir J. 2022;15;60:2103054;
39. Voorham J, et al. Allergy. 2019;74:273–283;
40. Bleecker ER, et al. World Allergy Organ J. 2022;15:100726;
41. Canonica G. Biomarker relatability in the International Severe Asthma Network. Oral presentation at WAC, Lyon, France 12–14 December 2019 (Abstract OC35);
42. Tran TN, et al. Ann Allergy Asthma Immunol. 2016;116:37–42;
43. Kupczyk M, et al. Allergy. 2014;69:1198–1204;
44. Heatley H, et al. Eur Respir J. 2019;54:PA2712;
45. Menzies-Gow A, et al. J Allergy Clin Immunol. 2020;145(3):757-765;
46. Thomas D, et al. Eur Respir J. 2022;60(5):2102583.